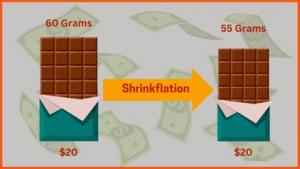
|
| ▲The first saliva self-diagnosis kit product, approved by Ministry of Food and Drug Safety, PCL Self-test COVID19 Ag (Source: Hankyoreh) |
As COVID 19 progresses, the Korean government has been managing confirmed patients by preventing the spread of COVID 19 by wearing masks, social distancing, and conducting vaccinations, while verifying whether they are confirmed by self diagnosis kits and/or PCR tests. Among them, the self diagnosis kit has the advantage of being able to be obtained without special procedures at nearby pharmacies or convenience stores, so it is highly accessible, allowing you the ability to check the results immediately with your eyes in a short time. Unlike the nasal rapid antigen test, which extracts specimens from the nose with a nasal swab, the saliva self diagnosis kit, which uses saliva as a sample, is drawing attention with the approval of the Ministry of Food and Drug Safety.
Q. What is different from the existing self-diagnosis kit?
A: Unlike the existing nasal rapid antigen self diagnosis kit, which collects samples by entering the nasal cavity with a cotton swab, saliva is spit and is used as a sample. Therefore, the saliva self-diagnosis kit contains a paper funnel, instead of a cotton swab.
Q. How are individuals to use it?
A: First, assemble the paper funnel and insert it into the solution container. After collecting saliva for 30 seconds, the user can spit into the solution container until the marked line. At this time, do not mix sputum. Since sputum contains a lot of waste, it is less accurate, so gather only clear saliva and spit it out. After collecting saliva, remove the funnel, close the solution container with a lid, and gently turn it up and down 10 times to mix the contents. Then, drip 3 droplets of the solution onto the test kit. After 10 minutes, check the results with your eyes. At this time, if it exceeds 20 minutes, the reliability of the test decreases, so the results should be checked between the 10 and 20 minute time period. When one red line appears, it is negative, and when two red and black lines appear, it is positive.
Q. What is the accuracy?
A: The Ministry of Food and Drug Safety only permits products with a sensitivity of 90% or more and specificity of 99% or more. Here, sensitivity is the probability of indicating a person with COVID 19 as positive, and specificity refers to the probability of indicating a personwithout COVID 19 as negative. The saliva self diagnosis kit approved by the Ministry of Food and Drug Safety this time is a, "PCL Self Test COVID19 Ag," product with a sensitivity of 94.29% and a specificity of 99.99%, the company said on its website.
Q. Where is the place of purchase and what is the price of this self-test?
A: From the 1st of this month, the government lifted distribution improvement measures for COVID 19 self diagnosis kits, allowing users to purchase saliva self diagnosis kits and COVID 19 self diagnosis kits, online. Therefore, it can be purchased at pharmacies, convenience stores, and online. According to a PCL official, the saliva self test COVID19 Ag, is the first licensed product of the saliva diagnosis kit, so it is estimated to be a little more expensive than the existing nasal rapid antigen self-diagnosis kit.
Q. What advantages are expected?
A: The nasal rapid antigen self diagnosis kit is not easy for adults to test because you have to push deep into your nose with a swab. Young children rejected the pain of the nasal examination, therefore, children, parents, and health workers have all struggled. Furthermore, there is a concern that the more often young children have swab enter their noses, the weaker the mucous membrane becomes, and it is dangerous. The saliva self diagnosis kit is expected to solve the disadvantages of this nasal rapid antigen self-diagnosis kit.
The saliva self diagnosis kits that improve the shortcomings of existing self diagnosis kits and the release of distribution improvement measures, will enable online purchase of self-diagnosis kits as a way to lower public anxiety, amid the lifting of outdoor masks. In addition, the launch of saliva self-diagnosis kits, could signal the development of various self-diagnosis methods and disinfection in the future. As everyone is trying to prevent COVID-19, individuals should also reamin alert, and thoroughly disinfection accordingly with the appropriate use of self-diagnosis kits.
By Kim Su-gyeong, cub-reporter tnrud2824@naver.com
<저작권자 © The Campus Journal, 무단 전재 및 재배포 금지>
 Shrinkflation, Consumer Deception
Shrinkflation, Consumer Deception